Avian Hemotology
by Rosalie Lane , AHT
The first step in critically analyzing a blood smear or cytology is the quality of the smear and stain. These are techniques you can learn everyday in the lab. You need an even smear with no smudge cells. The stain should be fresh (changed every 2 to 4 weeks) in order to provide the right cell colors. Adequate staining techniques should be second hand to you now. 20-30 dips in each diff quick stain cup are required.
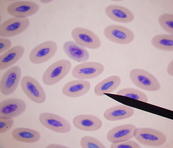
Erythrocytes (Red Blood Cells)
Assessment of RBCs provides information concerning possible anemia problems and / or bone marrow response (or lack there of). In Maturity, these cells are oval in shape, with a centrally placed nucleus that is comprised of evenly distributed chromatin clumping and a homogeneous stained cytoplasm regardless of the stain used. The majority of the representative RBCs on the differential should be mature. There should also be a small percentage of immature erythrocytes noted when evaluating the blood picture which denotes the “normal” replenishment of the RBC line. Immature erythrocytes seen serving this function are slightly larger than the mature RBC, having lightly stippled cytoplasm, and the chromatin clumping within the nucleus appears more loosely constructed. Degenerative RBCs appear smaller in size as does their nucleus, and the chromatin clumping within the nucleus is much more dense.
Polychromasia (color variation between RBCs) and anisocytosis (size variation between RBCs) are reported as follows:
An accurate PCV and TP should be obtained. Using a standard microhematocrit tube, centrifuge at 12,000 G for 5 minutes.
Assessment of RBCs provides information concerning possible anemia problems and / or bone marrow response (or lack there of). In Maturity, these cells are oval in shape, with a centrally placed nucleus that is comprised of evenly distributed chromatin clumping and a homogeneous stained cytoplasm regardless of the stain used. The majority of the representative RBCs on the differential should be mature. There should also be a small percentage of immature erythrocytes noted when evaluating the blood picture which denotes the “normal” replenishment of the RBC line. Immature erythrocytes seen serving this function are slightly larger than the mature RBC, having lightly stippled cytoplasm, and the chromatin clumping within the nucleus appears more loosely constructed. Degenerative RBCs appear smaller in size as does their nucleus, and the chromatin clumping within the nucleus is much more dense.
Polychromasia (color variation between RBCs) and anisocytosis (size variation between RBCs) are reported as follows:
- Slight – approx 5 to 10 polychrome cells per oil field
- Moderate – approx 10 to 20 polychrome cells per field
- Heavy – approx 40 to 50 % of RBCs are at least slightly polychrome
An accurate PCV and TP should be obtained. Using a standard microhematocrit tube, centrifuge at 12,000 G for 5 minutes.
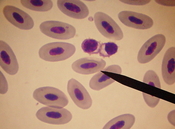
Thrombocytes
Avian thrombocytes, although their appearance is much different from the mammal platelet, they perform primarily the same function as their mammalian counterpart. Other thrombocyte functions have been speculated on, such as their possessing the ability to phagocytize foreign material in overwhelming situations, their response (or reactivity) to outside stimulus, and lastly, the possibility that they might be able to replace RBCs in cases of extreme anemias.
The avian thrombocyte is oval in shape with light blue to colorless cytoplasm containing approximately two to four small reddish granules usually at one end of the cell (called the pole). The nucleus stains very darkly and rarely has visible clumps. These cells are usually clumped together on the blood smear in groups of two, three, four, or more. These cells can be of help to the lab technician performing a differential in two ways:
Avian thrombocytes, although their appearance is much different from the mammal platelet, they perform primarily the same function as their mammalian counterpart. Other thrombocyte functions have been speculated on, such as their possessing the ability to phagocytize foreign material in overwhelming situations, their response (or reactivity) to outside stimulus, and lastly, the possibility that they might be able to replace RBCs in cases of extreme anemias.
The avian thrombocyte is oval in shape with light blue to colorless cytoplasm containing approximately two to four small reddish granules usually at one end of the cell (called the pole). The nucleus stains very darkly and rarely has visible clumps. These cells are usually clumped together on the blood smear in groups of two, three, four, or more. These cells can be of help to the lab technician performing a differential in two ways:
- These cells, as stated above, have the ability to become very reactive to chronic conditions and stimuli, thus denoting a chronic condition.
- By observing the aggregates of thrombocytes, one may be able to evaluate the value or quality of the slide submitted and thus take into consideration when estimates and counts of the representative cells are performed.
Granulocytes
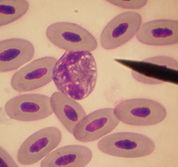
Heterophils
The avian heterophil which is most commonly compared to that of the neutrophil in mammals is the most frequently seen granulocyte in all species. As with the neutrophil, the heterophils are instrumental in body defense with incredibly large numbers available as well as their ability to phagocytize foreign bodies, bacteria, etc.
The avian Heterophil is generally circular-shaped with a bilobed nucleus. This may or may not be very visible through the rod-shaped granules contained in the colorless to faint blue cytoplasm. Depending on the species, there can be quite a variation of rod sizes and shapes and also whether the cytoplasm is colorless or faint blue. Using the recommended stain, the rods should stain a muddy pink in most species, and the cytoplasm will stain colorless to uneven variations of pink in cases of moderate to heavy degranulation. Many times in cases of high WBC counts, heterophils with very young granules (blue in color) and nuclei that are either band cells or cells with band-like nuclei would indicate that in response to outside insult or stimulus, the bone marrow is releasing cells before they mature. This is a very important sign and one to watch for and duly note with each differential performed.
The avian heterophil which is most commonly compared to that of the neutrophil in mammals is the most frequently seen granulocyte in all species. As with the neutrophil, the heterophils are instrumental in body defense with incredibly large numbers available as well as their ability to phagocytize foreign bodies, bacteria, etc.
The avian Heterophil is generally circular-shaped with a bilobed nucleus. This may or may not be very visible through the rod-shaped granules contained in the colorless to faint blue cytoplasm. Depending on the species, there can be quite a variation of rod sizes and shapes and also whether the cytoplasm is colorless or faint blue. Using the recommended stain, the rods should stain a muddy pink in most species, and the cytoplasm will stain colorless to uneven variations of pink in cases of moderate to heavy degranulation. Many times in cases of high WBC counts, heterophils with very young granules (blue in color) and nuclei that are either band cells or cells with band-like nuclei would indicate that in response to outside insult or stimulus, the bone marrow is releasing cells before they mature. This is a very important sign and one to watch for and duly note with each differential performed.
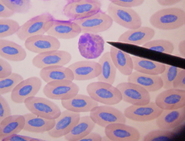
Eosinophils
Eosinophils are also circular in shape and contain granules in their cytoplasm. The staining characteristics of this cell appear to hinge on the type of stain used and the species of bird we’re dealing with. In our lab, the granules of the cockatiels, African Greys, lovebirds, and raptors, for example, do frequently stain a faint pink color. (This color is not comparable to the heterophil which is good). With these species, the cytoplasm will often be abundantly filled with granules, causing difficulty with identification. Other species such as cockatoos, Amazons, macaws, etc., seem to stain colorless to faint blue, but are recognizable due to the shapes of their granules. With both groups the nuclei stain much more intensely than the heterophil nuclei.
Eosinophils are typically associated with allergic reactions and parasitic infections and are rarely seen in high numbers except in raptor species.
Eosinophils are also circular in shape and contain granules in their cytoplasm. The staining characteristics of this cell appear to hinge on the type of stain used and the species of bird we’re dealing with. In our lab, the granules of the cockatiels, African Greys, lovebirds, and raptors, for example, do frequently stain a faint pink color. (This color is not comparable to the heterophil which is good). With these species, the cytoplasm will often be abundantly filled with granules, causing difficulty with identification. Other species such as cockatoos, Amazons, macaws, etc., seem to stain colorless to faint blue, but are recognizable due to the shapes of their granules. With both groups the nuclei stain much more intensely than the heterophil nuclei.
Eosinophils are typically associated with allergic reactions and parasitic infections and are rarely seen in high numbers except in raptor species.
Basophils
The easiest granulocyte to recognize, tend to be basophils. When stained, the granules of the basophil become a reddish-purple color. The basophilic granules are usually much smaller than the granules of the eosinophil. The cytoplasm stains a colorless to a light purple-red color and the nucleus doesn’t stain as intensely as the eosinophil. In cases of extreme toxicity, heterophils have been found to have very basophilic granules also. To avoid counting a toxic heterophil as a basophil, notice the darker staining nucleus of the basophil and the absence of cytoplasmic rods. Usually the cell with the very basophilic granules will be a basophil.
Basophils are seen in high percentages with finches, canaries, and smaller birds and are most of the time associated with chronic, long term illnesses.
The easiest granulocyte to recognize, tend to be basophils. When stained, the granules of the basophil become a reddish-purple color. The basophilic granules are usually much smaller than the granules of the eosinophil. The cytoplasm stains a colorless to a light purple-red color and the nucleus doesn’t stain as intensely as the eosinophil. In cases of extreme toxicity, heterophils have been found to have very basophilic granules also. To avoid counting a toxic heterophil as a basophil, notice the darker staining nucleus of the basophil and the absence of cytoplasmic rods. Usually the cell with the very basophilic granules will be a basophil.
Basophils are seen in high percentages with finches, canaries, and smaller birds and are most of the time associated with chronic, long term illnesses.
Mononuclear Cells
Lymphocytes and monocytes comprise this group of white blood cells in exotics as well as mammals. Since these cells do not have granules, they can be difficult to differentiate, especially if the stain used does not give good clarity and definition to the cell nucleus.
Lymphocytes and monocytes comprise this group of white blood cells in exotics as well as mammals. Since these cells do not have granules, they can be difficult to differentiate, especially if the stain used does not give good clarity and definition to the cell nucleus.
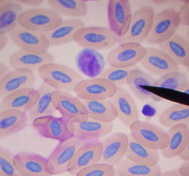
Lymphocytes
The most numerous of the white blood cells are lymphocytes and heterophils. Some species are naturally lymphocyte and there are some disease states that can cause a severe lymphocytic increase meaning more lymphs than hets would be seen. In some cases especially in Amazons, lymphocyte counts as high as 85% have been noted. After treatment, high counts remained from 60-65%. There are two different types of lymphocytes, small and large. The small lymphocyte is most commonly seen. They have the same characteristics with the round nucleus and chromatin clumps. The lymphocyte cytoplasm in generally light blue or blue stippled; the small lymphocyte and the large lymphocyte have a large nuclear/cytoplasmic ratio. Sometimes you can see lymphocytes so small that they may be difficult to identify from the thrombocytes. Keep in mind the different cytoplasmic colors (thrombo = usually clear to blue-gray; lymphocyte = colorless to light blue), the small granules in the pole of the thrombocyte, and the characteristic chromatin clumping in the nucleus of the lymphocyte. Lymphocytes can become so reactive that the cytoplasm have faint pink tinges, cytoplasmic blebbing can occur on the borders of the cell, and less chromatin clumping is noted in the nucleus. When these signs in lymphocytes are present, it can be harder to identify them comparing them to monocytes. Bacterial infection can be correlated to slight to moderately reactive lymphs where as moderate to heavily reactive lymphs have been most often associated with viral conditions including PBFD, neoplasia, such as lymphoma or lymphosarcoma, as well as other viral problems.
The most numerous of the white blood cells are lymphocytes and heterophils. Some species are naturally lymphocyte and there are some disease states that can cause a severe lymphocytic increase meaning more lymphs than hets would be seen. In some cases especially in Amazons, lymphocyte counts as high as 85% have been noted. After treatment, high counts remained from 60-65%. There are two different types of lymphocytes, small and large. The small lymphocyte is most commonly seen. They have the same characteristics with the round nucleus and chromatin clumps. The lymphocyte cytoplasm in generally light blue or blue stippled; the small lymphocyte and the large lymphocyte have a large nuclear/cytoplasmic ratio. Sometimes you can see lymphocytes so small that they may be difficult to identify from the thrombocytes. Keep in mind the different cytoplasmic colors (thrombo = usually clear to blue-gray; lymphocyte = colorless to light blue), the small granules in the pole of the thrombocyte, and the characteristic chromatin clumping in the nucleus of the lymphocyte. Lymphocytes can become so reactive that the cytoplasm have faint pink tinges, cytoplasmic blebbing can occur on the borders of the cell, and less chromatin clumping is noted in the nucleus. When these signs in lymphocytes are present, it can be harder to identify them comparing them to monocytes. Bacterial infection can be correlated to slight to moderately reactive lymphs where as moderate to heavily reactive lymphs have been most often associated with viral conditions including PBFD, neoplasia, such as lymphoma or lymphosarcoma, as well as other viral problems.
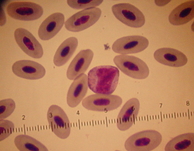
Monocytes
Most of the time monocytes are the least numerous and the largest of white blood cells. They appear to have no consistent shape to the cytoplasm and are large in size and cytoplasmic nuclear ratio. The cytoplasm color ranges from a dull blue-gray (ground glass appearance) to a blue-gray with some pink tinged areas. The monocyte nucleus is not round like the lymphocyte. It can range from a bean-shape to a very indefinite shape with a fine, lacy reticular chromatin pattern. Criteria used to determine lymphocytes from monocytes are:
For years the issue of monocyte vs. lymphocyte has lived on and until some extensive EM studies are done or a specialized training technique is found the issue will be unlikely resolved.
Depending on the species, it can be very difficult to show or describe one or two examples of each type of white blood cell. Heterophils and eosinophils can look very different.
Most of the time monocytes are the least numerous and the largest of white blood cells. They appear to have no consistent shape to the cytoplasm and are large in size and cytoplasmic nuclear ratio. The cytoplasm color ranges from a dull blue-gray (ground glass appearance) to a blue-gray with some pink tinged areas. The monocyte nucleus is not round like the lymphocyte. It can range from a bean-shape to a very indefinite shape with a fine, lacy reticular chromatin pattern. Criteria used to determine lymphocytes from monocytes are:
- Nuclear chromatin clumping (or lack of)
- Appearance of the cytoplasm for color, volume, and consistency.
For years the issue of monocyte vs. lymphocyte has lived on and until some extensive EM studies are done or a specialized training technique is found the issue will be unlikely resolved.
Depending on the species, it can be very difficult to show or describe one or two examples of each type of white blood cell. Heterophils and eosinophils can look very different.
White Blood Cell Count Determinations
There are several methods for determining the white blood cell count in birds. The method chosen is highly dependant on the method chosen as well as the facility.
Eosinophil Unopette Method
Actual counts of the avian granuloctyes (heterophils, eosinophils, and basophils) by means of an Eosinophil Unopette manufactured by Becton-Dickinson comprise this method. A dilution of blood is prepared in the Unopette containing phloxine dye and is allowed to sit for approximately 5-10 minutes. Both chambers of a hemacytometer are then charged with the solution and the dark pink or red cells (representative granuloctyes) are counted. This number is then multiplied by 1.1 (+/- 10%); then by 16 and the final number is referred to as the granulocyte count. Since this method allows only for the granulocytes being counted, adjustments must be made for the mononuclear cells (monocytes and lymphocytes) and is accounted for from the differential; the granulocyte count in divided by the total number referred to as the Corrected White Blood Cell Count. This method (although probably the best actual counting method for avian blood), has several aspects which require consideration.
Accuracy of the test is the size and state of the patient. It is this author’s experience that the durability of the granulocytes is directly proportionate to the size and/or health (or lack of) of the bird. Many times the granuloctyes of these two groups rupture all too easily and will definitely affect the accuracy of this test.
Given the right circumstances, there are a few other methods for counting actual counts, but are also limited to time taken collecting the sample due to clotting factors.
Estimated White Blood Cell Counts
This method, for the author, has been found to be the most consistently reliable.
To begin, simply scan the stained blood smear on low power (10x) to check the distribution of the cells and focus on high dry (40x) in an area found to be evenly distributed. Then count the white blood cells found in ten evenly distributed fields. As a general rule, do not use fields where clumps of white blood cells found in those ten fields. Divide by 10, thus finding the average number per field. Multiply this number by 2,000 and report the number incorporating a range value. For example:
Total White Blood Cells Counted in Ten Fields : 165.
Divided by 10 (# of fields counted) : 16.5
Multiplied by 2 : 33 .
Estimated White Blood Cell Count w/o Range : 33,000.
Cases with a white blood cell count in the 140,000 and up range were found primarily with Mycobacteria sp. Cases. In the case of the example given above, the range would be 31,000 to 35,000. In more normal numbers an estimated white blood cell count of 17,000 would be expressed as 16-18 thousand.
This method has both positive attributes and limitations associated with it.
So, practice in lab. Ask lots of questions!
Eosinophil Unopette Method
Actual counts of the avian granuloctyes (heterophils, eosinophils, and basophils) by means of an Eosinophil Unopette manufactured by Becton-Dickinson comprise this method. A dilution of blood is prepared in the Unopette containing phloxine dye and is allowed to sit for approximately 5-10 minutes. Both chambers of a hemacytometer are then charged with the solution and the dark pink or red cells (representative granuloctyes) are counted. This number is then multiplied by 1.1 (+/- 10%); then by 16 and the final number is referred to as the granulocyte count. Since this method allows only for the granulocytes being counted, adjustments must be made for the mononuclear cells (monocytes and lymphocytes) and is accounted for from the differential; the granulocyte count in divided by the total number referred to as the Corrected White Blood Cell Count. This method (although probably the best actual counting method for avian blood), has several aspects which require consideration.
- Time limitations: The blood and phloxine dilution should not sit over ten minutes causing significant amounts of the dye being taken up by cells other than granulocytes making an accurate count almost impossible. Time restrictions can be very limiting as well as blood clotting in the hematocrit tubes. Being able to draw blood from the toe nail and counting it quickly could make this a very useful method in most instances.
- Differential Dependence: This method can also be affected by the differential count which is used to calculate for the corrected white blood cell count. The extent to which this method is affected is dependant on the technician’s avian hematology experience.
- Size and state of the Bird: Other conditions affecting to varying degrees the
Accuracy of the test is the size and state of the patient. It is this author’s experience that the durability of the granulocytes is directly proportionate to the size and/or health (or lack of) of the bird. Many times the granuloctyes of these two groups rupture all too easily and will definitely affect the accuracy of this test.
Given the right circumstances, there are a few other methods for counting actual counts, but are also limited to time taken collecting the sample due to clotting factors.
Estimated White Blood Cell Counts
This method, for the author, has been found to be the most consistently reliable.
To begin, simply scan the stained blood smear on low power (10x) to check the distribution of the cells and focus on high dry (40x) in an area found to be evenly distributed. Then count the white blood cells found in ten evenly distributed fields. As a general rule, do not use fields where clumps of white blood cells found in those ten fields. Divide by 10, thus finding the average number per field. Multiply this number by 2,000 and report the number incorporating a range value. For example:
Total White Blood Cells Counted in Ten Fields : 165.
Divided by 10 (# of fields counted) : 16.5
Multiplied by 2 : 33 .
Estimated White Blood Cell Count w/o Range : 33,000.
Cases with a white blood cell count in the 140,000 and up range were found primarily with Mycobacteria sp. Cases. In the case of the example given above, the range would be 31,000 to 35,000. In more normal numbers an estimated white blood cell count of 17,000 would be expressed as 16-18 thousand.
This method has both positive attributes and limitations associated with it.
- Many times complaints have been raised that using this method is very difficult to duplicate between technicians given slides from the client. This may be due to a lack of experience on the technician’s part and/or the quality of the smears made. It will take a novice technician a few weeks to a few months to feel reasonably comfortable with estimating. However, if the blood smears that are sent are poor quality, it will take even longer for the novice technician to learn. Even the experienced technician will take longer to read these types of slides and the accuracy may be affected as well.
- There are many advantages to of this method. First, sample preparation is easily performed since most veterinarians and staff members routinely make blood slides. This is not to say that good quality, equally distributed blood smears are routinely made but that it is quite possible for anyone to make good blood smears given adequate practice. Accuracy of the results is not affected with the clotting of blood in the hematocrit tubes as with the previous methods, thus eliminating possible problems with transfer of sample to outside laboratories.
So, practice in lab. Ask lots of questions!

